UDI stands for Unique Device Identification. The regulation (For manufactures no hospitals) establishes a unique device identification system for medical devices requiring the label of devices to bear a unique identifier. The unique identifier shall adequately identify the device through distribution and use, and may include information on the lot or serial number.
This Allows more accurate reporting, reviewing and analyzing of adverse event reports so that problem devices can be identified and corrected more quickly.
Reducing medical errors by enabling health care professionals and others to more rapidly and precisely identify a device and obtain important information concerning the characteristics of the device.
Enhancing analysis of devices on the market by providing a standard and clear way to document device use in electronic health records, clinical information systems, claim data sources and registries. A more robust postmarket surveillance system can also be leveraged to support premarket approval or clearance of new devices and new uses of currently marketed devices.
Fewer errors, accurate inventory, more efficiency in the entire distribution chain; but most importantly patient safety are a few reasons to mark your instruments. For example, when you have hundreds of Kerrisons and a particular one needs special attention (something as simple as a repair) it will be easier to locate it with its unique identification number & then to perform any corrective repair/replacement of the instrument. Imagine having a room full of people, but you really don’t know who they are, the people in that room today will be the same in that room tomorrow? For the same analogy, with your instruments marked, you know the people in the room because now they have a unique identification. The same instruments will be in that tray and you know with 100% accuracy which one goes where. DataMatrix is a matrix (2D or two-dimensional) barcode which may be printed as a square or That is a tough question, Margaret. It depends on the instrument we are marking. Most of the instruments we mark are aged/ out of warranty and some of them have already been repaired by third-party repair service. I think placing a 0.20mm X 0.20mm Datamatrix mark on an instrument will not affect any functionality on the instrument. But, it is always a good idea to consult with the instrument manufacturer to see if you are still under warranty. Passivation is the use of a light coat of protective material, such as metal oxide, to create a shell against corrosion. Many instruments have a natural coat to protect against free iron. There are many variables to break this coat with the laser mark. Our techniques and the mark's size won't alter or free iron from the metal, however, we have a valid way to test (ASTM A-967/A967M-13) free iron. When a failure happens, we can quickly re-passivate the instrument with approved natural products. Updated 09-28-2020. I read this topic many times on the internet. Basically, it is a claim with no support. No. Electrolysis is not a method we will use at NuTrace. Among many reasons the most relevant are: -It is a slow process to mark an average of 35,000 instruments in a 300-500 bed hospital. More information about this topic: No, not at all. These are two completely different technologies. RFID (Radio Frequency Identification) uses a small passive sensor glued to the instruments and requires a transmitter to excite the sensors to respond from a UHF signal. DataMatrix is a 2D barcode embedded to the instrument, never at risk for failure or even worse, becomes an URFOs (Unintended Retention Of Foreign Objects). Absolutely, we love to help and make things more affordable for our customers. Great, we cannot wait to do it. However, we need to have a better understanding of what you are trying to achieve. We need to know which tracking solution you have, how the sequence of numbers is designed, which instruments you are starting to mark, number of instruments, how they are input in the tracking system etc.. One important piece of information for us is your location. We can accommodate many solutions for you, but in order to make it more affordable to you and your hospital, we need to evaluate if is better for us to teach your tech(s) how mark the instruments with one of our lasers or for us to do it on site. Absolutely. Our Fiber laser meets the ANSI Z-136 guidance for laser safety. The laser's enclosure makes it a class 1 laser (Class IV inside) which is very safe to operate.You can find more information anout our NuTrace fiber laser NuTrace fiber laser here Thanks for your question Donna, absolutely, we can place a laser DataMatrix code on a cylindrical surgical instrument.
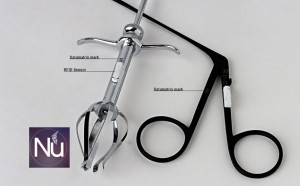
Marking Surgical Instruments
rectangular symbol made up of individual dots or squares. This representation is an ordered grid of
dark and light dots bordered by a finder pattern. The finder pattern is partly used to specify the orientation and structure of the symbol. The data is encoded using a series of dark or light dots based upon a predetermined size. The size of these dots is known as the X-dimension. The size of the code can be as small as 0.16.mm or 6.6.mil.
The size of the mark is adjusted according to the instrument been marked. (Eyes, Orthopedic, etc..)
Updated 09-28-2020. Marking your old instruments won't take away the warranty, keep in mind under the UDI rule new instruments are coming already marked, you need to learn and understand what the GS1 tells you so you will decide if another mark is needed or not. Contact us for further information
We have researched for many years the process of passivation and the interaction of the laser with surgical instruments, base on that research we had developed RATERO, a 2,5-second cleaning solution that will molecularly clean your instrument and removed microparticles that originate rust
Growing bacteria inside DataMatrix Laser Mark
-The Electrolysed solution is a chemical and not environmentally friendly.(Sodium chloride, copper sulfate, dilute sulfuric acid are examples of strong electrolytes).
-You cannot do plastics (Da Vinci instruments for example, or any anodized instrument)
-It is a very superficial mark so won't last long under the daily SPD process.
-You are limited to the mask size.
Click here
We want to remove any objects from the instruments, not to add another one.
Let us know what kind of laser you have, a little more information about your hospital, your project, your location and we will send you one of our CAD-Laser specialists. We will get you up and running, and if this makes you happy...we will do it for free!!
Contact us for more information.

The smallest mark we can place and still been read by our R+ is 800 microns
Setting Your Instruments in NuTrace
That is a great question. Tracking surgical instruments is a project in which we will work with you and your hospital from day one. If you want to track at the instrument level, we will mark your instruments and we will provide you with the right software to track in the entire supply chain. Plus, all the tools we have for the O.R and we will provide you with the right hardware to read & track those instruments (Laserless readers). It is like buying a car; we will give you the key and you can go and drive your car right away... rather than giving you the body and letting you go and find the tires, engine, seats, etc...
Yes, it will take 10 seconds to complete and NuTrace will create an alias for that instrument so we won't break the sequence we have with other instruments. Just scan them with the R+ and done. They will automatically be added to the database by NuTrace.
Absolutely NO! Everything built inside Nutrace is just one module (Reports, vendors, O.R planning, Implants, BOM, and much more). NuTrace is priced as a complete solution and we continue expanding, researching and making it more powerful for our customers at no additional cost. It is the same analogy of buying a car. We will sell to you an entire car and our price is fixed, we won't show you a car and then charge you for mirrors, seats, radio, trunk etc...
It requires a little analysis from our coders and developers. Most likely we will have to rebuild your database in order to make it NuTrace compatible but, it can be done. As far as hardware, we will use whatever we can to save you costs, (Computers, label printers etc) but most likely you will love and want to have our hardware.(Nano reader, Nu RFID Reader, R+). If you are at the tray level and want to move to the instrument level; that would be an easy transition, we just need to mark your existing surgical instruments. There is always the option for you to perform the marking and we will train your staff to do it. Please see the JESSA case study
Gee.... where do we start? Basically it comes down to knowledge and passion; all we do, think, talk, and plan is tracking instruments. What make us different from our competitors is how we see Sterile Processing. We see SPD as a production line, rather than just another department. We know the clients you have to manage every day in the O.R. We ourselves understand both needs because the people at NuTrace have been inside and part of these departments for years. We understand the problems, we look, listen, feel and bring solutions to those problems. The most important tool inside NuTrace is closing the miscommunication gap between OR and SPD.
Absolutely not. Our system is as many instruments as you want to add and as many trays as well. Same car analogy. The car dealer will charge you an additional fee for the car because you are carrying five passengers instead of two. With NuTrace, you can add whatever quantity of instruments you need/want.
Absolutely yes! Our philosophy at NuTrace is to provide speed for the competitive new healthcare environment and that includes SPD, not to make equipment proprietary. Our readers (Nano-R+) have proven to be efficient, faster, convenient and more affordable than other solutions. Not to mention they are the safest in the market. It is up to your tracking provider to let us interface with their tracking system.
Tracking Your Instruments
Thanks for your question. We do in fact specialize in very small marks! We have developed the R+, the fastest laserless 2D barcode reader in the world. It is not only impressive because of its speed, but it can also read codes the size no other reader can, and the price? The price is much lower than a conventional 2D handheld barcode reader.
Thanks for your question. You can have NuTrace open to use any reader you want however; everything comes down to what you are tracking at the moment. If you are at the tray level and you are reading the white labels (1D barcodes), your current readers (basic) will work, however keep in mind:
-1 When you move to track at the instrument level, marks will be very small 2D DataMatrix and they will be harder and more difficult to read with those expensive readers. This will result in your tech taking more time to assembly the tray (inefficient use of time trying to read the code).
-2 Check if the readers you have are Class II lasers. When you scan the instruments there is no white label (to absorb laser aim) on the instrument so it will reflect that laser back to the operator (safety concerns). See article.
-3 Our R+ is a Hospital grade reader (Stainless steel), far more affordable, significantly faster than your regular one and is hands free.
Allow us to present you with the options Nutrace has not available, not only the 2D readers but in the RFID as well (Only for trays...not for instruments).
The answer is no, you won't have problems with NuTrace because ours is one complete solution (On your mark, get set, Track!). Once implemented you won't have those problems because you will have all of the perfect solutions for the tracking.
I know and understand your frustrations, as we been there before while helping customers. The main problem starts with accountability of the "Software" manufacturers. They will sell you the software and whatever else they can fit in between, which will become your problem not theirs.
Tracking at the instrument level is a field we have already mastered. There are so many variables we must apply to every phase to make it perfect (Study the 2D marks, size,algorithms in the mark, size of the matrix, contrast, dimensional structure etc) and this is only for the DataMatrix. Logistics for the internal number, how many digits, how many letters, what separation, encoding methods, etc... and the marking techniques size material, etc....











10 Comments